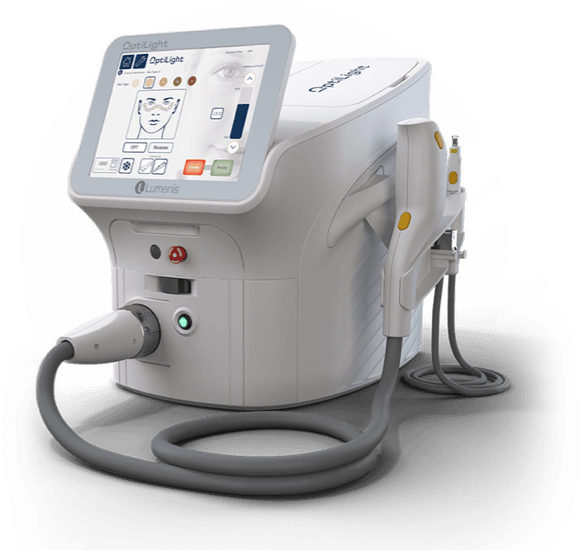
The First and Only IPL FDA Approved for Dry Eye Management
Stop the Dry Eye Vicious Cycle of Inflammation
Reduce Inflammatory Mediators
Decrease the level of pro-inflammatory mediators to inhibit inflammation.1,2
Improve Tear Breakup Time
Significantly boost tear break up time and decrease osmolarity.6,7
Alleviate Abnormal Blood Vessels
Destroy the abnormal blood vessels that perpetuate inflammation.3,4
Restore Meibomian Glands
Improve meibomian gland morphology and functionality.2
Decrease Demodex
Decrease the population of Demodex mites, which stimulate infection and boost the bacterial load on eyelids.5
Elevating Dry Eye Management
Every element designed to provide a precise and comfortable dry eye procedure





Lumenis’ Bright Solution: Backed by Peer-Reviewed Clinical Studies*
Effective multifactorial treatment for a multifactorial disease
Elevating Your Practice

Light-based, fast,
15-Minute Procedure
Each OptiLight session takes about 15 minutes, so you can easily integrate OptiLight into the workflow. With just 4 comfortable sessions, OptiLight is an essential tool in your dry eye toolkit.
Antares Dry Eye Disease Diagnostics
Advanced, automated DED diagnostic and corneal topographer
Applications
- Dry eye report
- Meibography
- Tear Breakup Time
- Tear Meniscus
- Topographer

Learn How You Can Elevate Your Practice
Download OptiLight Info Kit
Learn about the first and only IPL FDA approved for dry eye management

OptiLight Specifications
ExpertFilters*
OptiLight; Acne: (400-600 & 800-1200); Vascular (530-650 & 900-1200); 515 nm, 560 nm; 590 nm; 615 nm; 640 nm; 695 nm; 755nm;
Lightguides
15x35mm, 8x15mm, 6.4mm Ø
Maximal Fluence**
35 J/cm2
Cooling
Continuous contact cooling for IPL handpiece
* OptiLight filter is received complementary with the system. Other filters can be purchased separately
** For upgraded IPL aesthetic configuration
1. Liu et al. (2017), Am J Ophthalmol 183-190; 2. Yin et al. (2018), Curr Eye Res 43(3):308-313; 3. Kassir et al. (2011), J Cosmet Laser Ther 13(5):216-322; 4. Papageorgiou et al. (2008), Br J Dermatol 159(3):628-632; 5. Prieto et al. (2002), Lasers Surg Med 30(2):82-85; 6. Dell et al. (2017) Clin Ophthalmol 11:817-827; 7. Toyos & Briscoe (2016), J Clin Exp Ophthamol 7:6.
* All clinical studies cited here were conducted with Lumenis IPL with OPT technology.
OptiLight is intended to be used by licensed practitioners, according to local rules and regulations.
Risks and warning (non-inclusive list):
Indication for Use: Improvement of signs of Dry Eye Disease (DED) due to Meibomian Gland Dysfunction (MGD), also known as evaporative dry eye or lipid deficiency dry eye, in patients 22 years of age and older with moderate to severe signs and symptoms of DED due to MGD and with Fitzpatrick skin types I-IV. IPL is to be applied only to skin on the malar region of the face, from tragus to tragus including the nose (eyes should be fully covered by protective eyewear). IPL is intended to be applied as an adjunct to other modalities, such as meibomian gland expression, artificial tear lubricants and
warm compresses.
Treatment with OptiLight is contraindicated for patients with the following conditions in the treatment area: Ocular surgery or eyelid surgery or Neuro-paralysis within 6 months prior to the first treatment; Uncontrolled eye disorders affecting the ocular surface; Pre-cancerous lesions, skin cancer or pigmented lesions; Uncontrolled infections or uncontrolled immunosuppressive diseases; Recent Ocular infections; History of cold sores or rashes in the perioral area, including: Herpes simplex 1 & 2, Systemic Lupus erythematosus and porphyria; Use of photosensitive medication and/or herbs that may cause sensitivity within 3 months prior to the first IPL session; Recent radiation therapy to the head or neck or planned radiation therapy; Recent treatment with chemotherapeutic agent or planned chemotherapy; History of migraines, seizures or epilepsy. Patients eyes must be completely occluded during the treatment. Please refer to the operator manual for a complete list of intended use, contraindications and risks.
The following possible side effects can occur following IPL treatments: Pain/discomfort, damage to natural skin texture, change of pigmentation, scarring, excessive edema, fragile skin, bruising, burns, pruritus and xerosis. Please refer to the user manual or ask your doctor for a complete list of intended use, contraindications and risks.





Reviews
There are no reviews yet.